Interdisciplinary research to identify causal biology of human cancers
We employ a translational approach—investigating disease in humans, validating insights in models, then returning to human studies—to uncover critical factors that connect the DNA damage response to anti-tumor immunity. Our aim is to translate discoveries into treatments for patients in the clinic. See our lab's main research areas below.
Current Research Areas
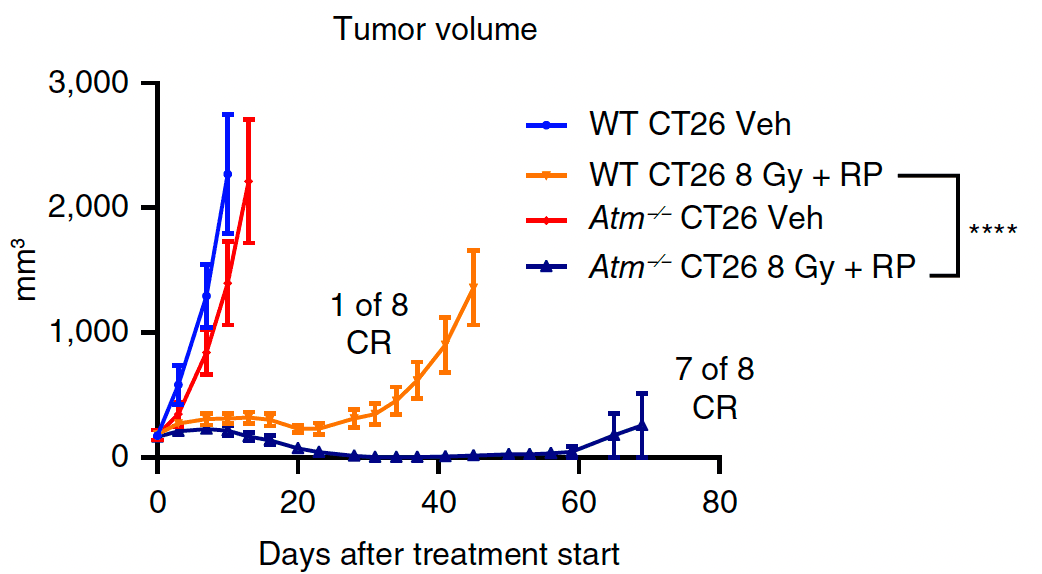
01Homologous recombination deficiency and anti-tumor immunity
Using translational data from human tumors and complementary model systems, we show that homologous recombination deficiency reshapes antigen presentation and innate signaling in unique ways depending on the affected gene.
Ongoing work dissects the mechanistic basis for these observations to pinpoint DDR-immunity interactions that can be exploited therapeutically.
Key references: Samstein et al., 2021, Sinha et al., 2025.

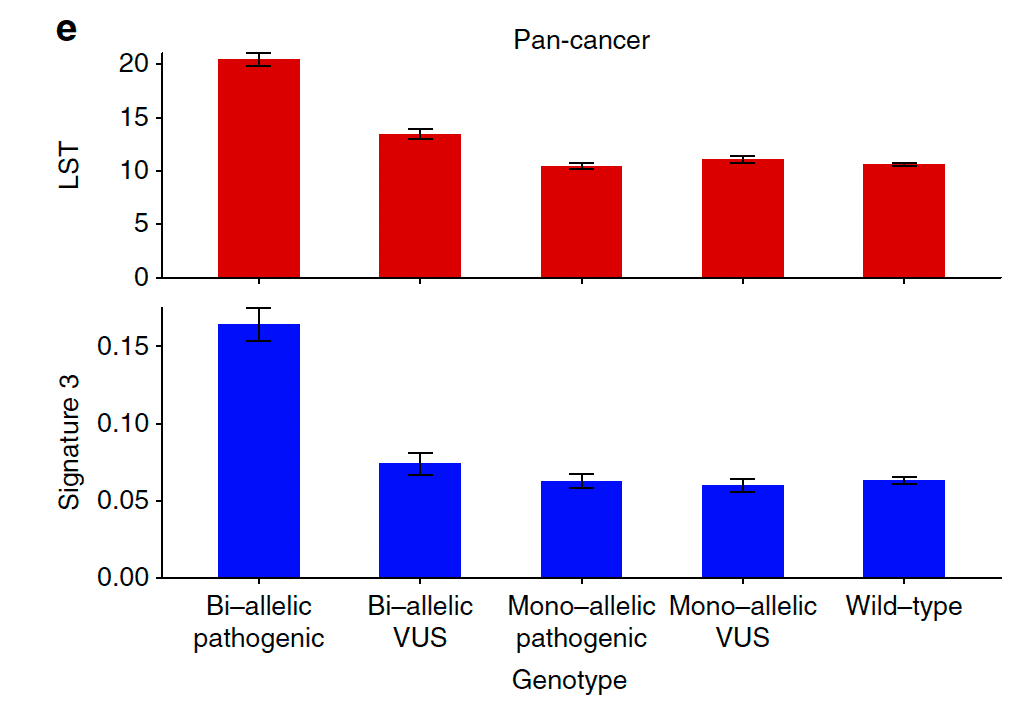
02Computational analysis of DNA repair
Patterns of mutations in cancer genomes reveal homologous recombination deficiency as a pan-cancer phenotype and expose DNA repair liabilities that can be targeted with precision therapy.
We collaborate to identify synthetic viable partners for HRD tumors and to chart genome-wide markers of sensitivity to cytotoxic regimens, extending these atlases with new cohorts and curated pipelines.
Key references: Riaz et al., 2017, Pitter et al., 2021, Zhu et al., 2024.
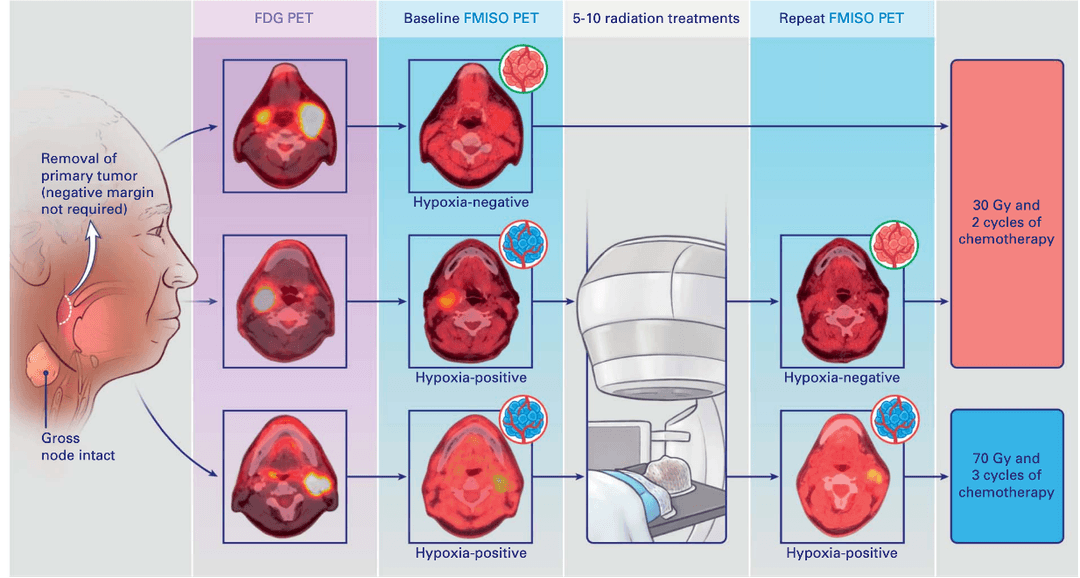
03Precision radiotherapy for HPV-positive OPC
Prospective trials and correlative studies demonstrate that carefully selected HPV-positive oropharyngeal cancer patients can achieve excellent control with de-intensified regimens.
Current efforts probe hypoxia dynamics, tumor genome structure, and ctDNA kinetics to personalize radiotherapy dosing strategies for each patient.
Key references: Riaz et al., 2021, Lee et al., 2024.
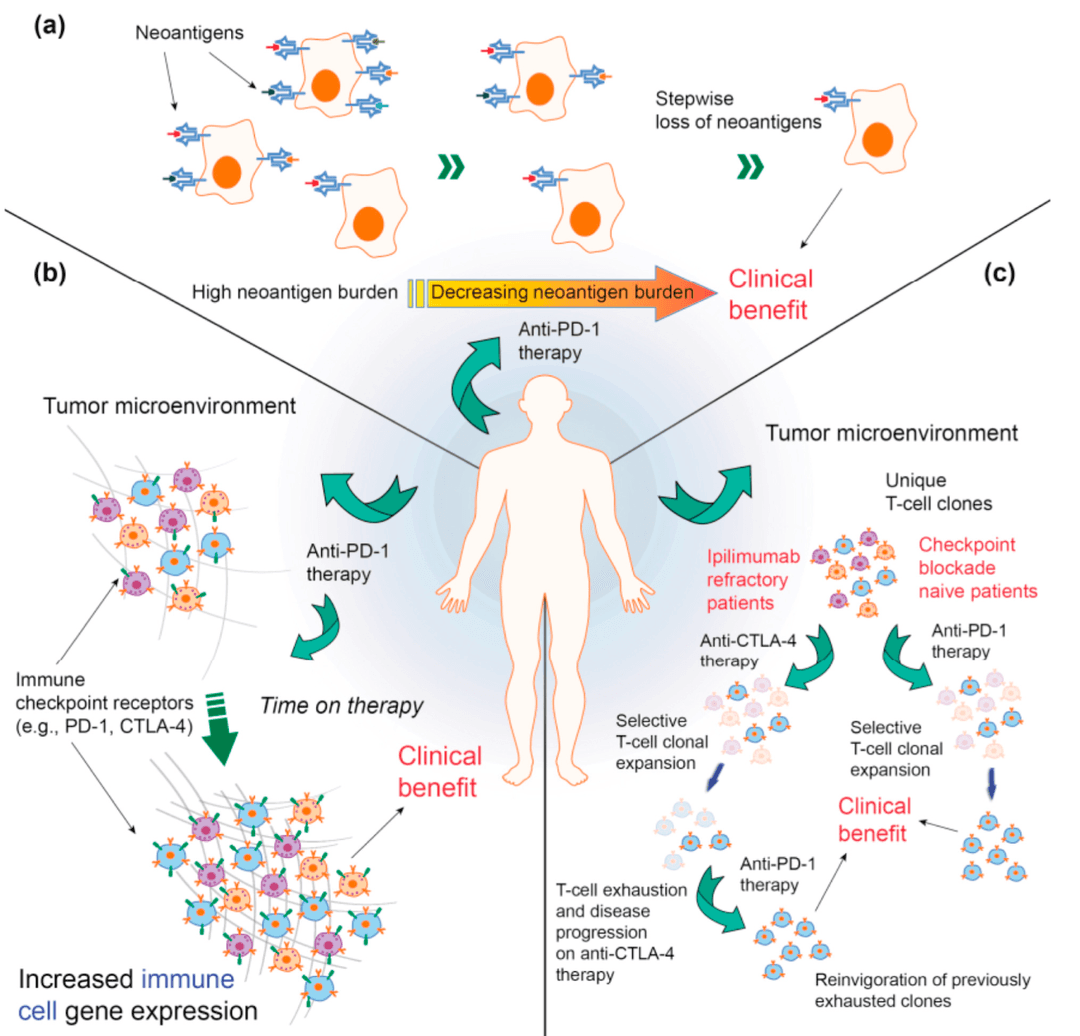
04Immunogenomics
Paired tumor and blood profiling shows that T-cell clones expand in proportion to tumor mutation burden and that immune checkpoint therapy drives neoantigen-specific editing.
Together with collaborators we have identified neoantigen-reactive clones responsible for these effects; current work explores additional immunogenomic features that influence response and resistance.
Key references: Riaz et al., 2017, Alban et al., 2024.
05Translational radiotherapy
Rather than one-size-fits-all trials, we identify genotype-specific combinations in preclinical systems and bring the most promising strategies into biomarker-rich clinical studies.
With collaborators, we used ctDNA to distinguish radiotherapy effects in oligoprogressive disease, separating truly oligoprogressive tumors from those with systemic progression.
Key references: Ng et al., 2021, Tsai et al., 2024.